Installation & Training | Learn with Us
Access professional training and installation tutorials to easily configure, commission, and operate our systems. Whether you're a technician or an end-user, you'll find practical and valuable learning resources here.
In this video, we walk you through the full installation process of the Xtreme LV system.

Welcome to Renon Power’s installation guide for the ECube 60AP battery storage system!
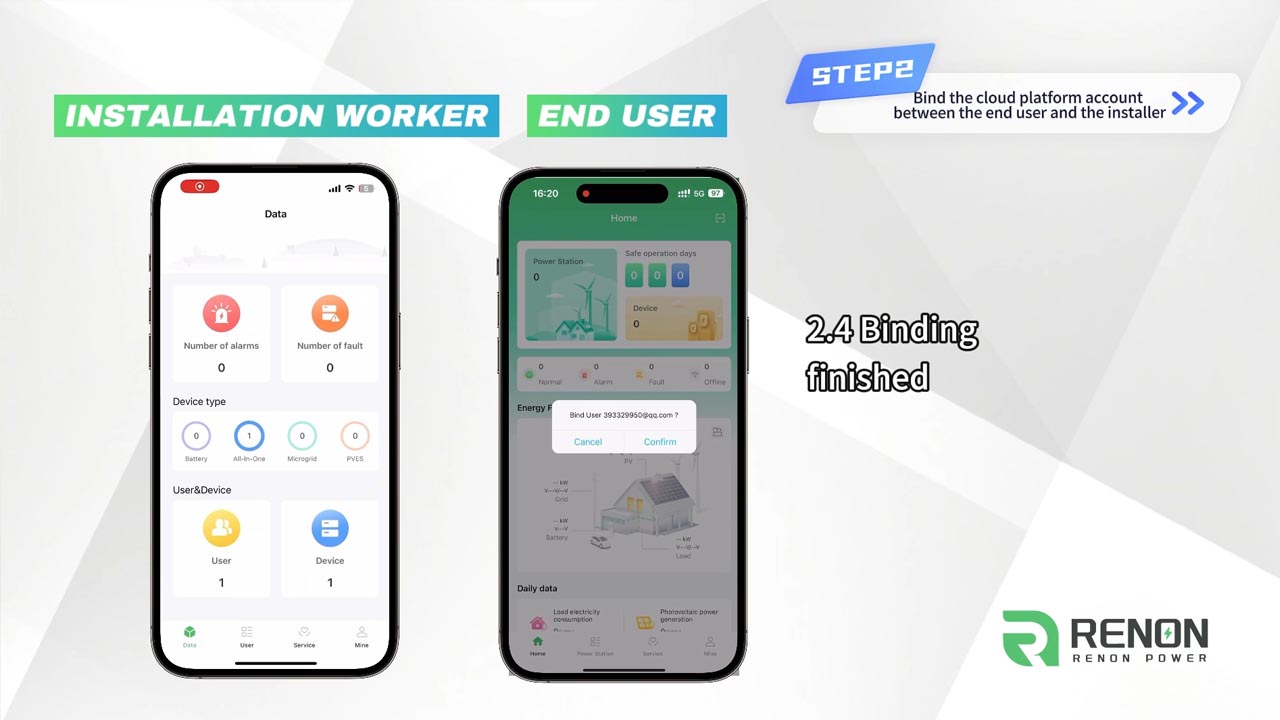
Renon’s new cloud platform takes energy management to the next level with its advanced network configuration features.